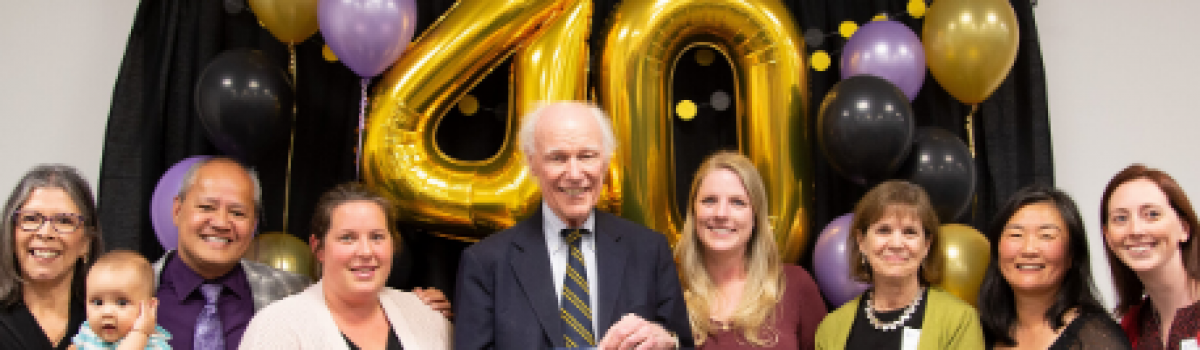The very first MotherToBaby information service is celebrating its 40th year of service at the UC San Diego Center for Better Beginnings this October! Program Founder and current Medical Director Dr. Ken Lyons Jones recalled that when he joined UC San Diego in 1974 as a new pediatrician, he was fresh off the publication of his seminal paper that first described Fetal Alcohol Syndrome, a condition that results in brain damage and growth problems in a child when alcohol is consumed during pregnancy. As a specialist focused on identifying and treating children with birth defects, Dr. Jones noticed that he started receiving an increasing number of calls from his healthcare colleagues and from women in the local community who wanted to know about the effects of different environmental exposures (like medications a mom was taking) during pregnancy and while breastfeeding.
“What we’d do was say, ‘We don’t know, but we could find out!’ Then we’d race over to the library and look up everything that we could find on that drug. We’d find all kinds of information about the effects of the medication on a rat, guinea pig or mouse – and we would call back the woman and tell her everything that we found out about the effects on that rat, guinea pig and mouse. And she’d say ‘that is really interesting – but what about me?’ And we’d say, unfortunately there’s no information about how it affects human health or developing fetus,” said Dr. Jones while addressing the audience at our October 3rd celebration. The volume of calls he received suggested to Dr. Jones a dire need in the community, so in 1979 Dr. Jones founded the first “teratogen information service” – a service dedicated to providing up-to-date, evidence-based information about environmental exposures during pregnancy and while breastfeeding. Initially called the San Diego County Teratogen Registry, the program soon expanded organically – and by necessity – into a parallel research program to collect pregnancy information from women with select exposures so that critical information gaps on medication safety could be filled.
Over the years, the name of the service changed a few times. In 1989, it became the California Teratogen Information Service (CTIS) and Clinical Research Program. Later, the program became an affiliate of the non-profit Organization of Teratology Information Specialists (OTIS), and when OTIS changed the name of the information service to MotherToBaby in 2013, MotherToBaby California was born! Similarly, the research program has evolved over time and is now known as MotherToBaby Pregnancy Studies, one of the largest pregnancy registries on medicines and vaccines in North America.
As the first-of-its-kind, MotherToBaby California has been used as a model nationally and worldwide for the development of other teratogen information services. Importantly, in its 40 year history the program has always been available at no-cost to the public. It has been supported by continuous funding since 1981 from the California Department of Education, and since 2014 has also received sub-awardee funding from the U.S. Health Resources & Services Administration.
Earlier this month, moms, community partners, and current and former program staff came together to celebrate the monumental achievements of the program. The value of the program, as one mom shared, was that “While there were a lot of unknowns about my medications, I at least felt like I had some information and I could make an educated decision – not one based on fear.” Please join us in celebrating the origins and accomplishments of MotherToBaby California and our continued effort to promote the health of moms and babies and reduce the risk of preventable birth defects!
Learn More
 Your Gift Supports Critically-Needed Research On The Effects Of COVID-19 Infection On Mom And Baby
Your Gift Supports Critically-Needed Research On The Effects Of COVID-19 Infection On Mom And Baby 



 As part of the National Institute of Alcoholism and Alcohol Abuse (NIAAA)-funded
As part of the National Institute of Alcoholism and Alcohol Abuse (NIAAA)-funded  With Dr. Wertelecki and the Principal Investigators in Ukraine, Dr. Lyubov Yevtushok and Dr. Natalya Zymak-Zakutnya, findings from these research activities have helped inform interventions. As a result, members of the research team have engaged in trainings for clinicians across Ukraine, and have partnered with OMNI-Net to develop the first
With Dr. Wertelecki and the Principal Investigators in Ukraine, Dr. Lyubov Yevtushok and Dr. Natalya Zymak-Zakutnya, findings from these research activities have helped inform interventions. As a result, members of the research team have engaged in trainings for clinicians across Ukraine, and have partnered with OMNI-Net to develop the first